Abstract
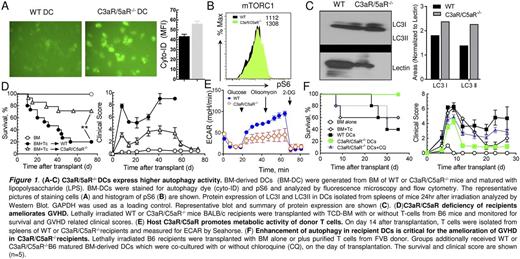
Graft-versus-host disease (GVHD) limits the success of allogeneic hematopoietic stem cell transplantation (allo-HCT). Traditional therapies have targeted T cells, yet immunostimulatory dendritic cells (DCs) are critical in the pathogenesis of GVHD. Autophagy is a self-degradative process for cytosolic components and is required for maintaining homeostasis after allo-HCT. Complement receptors C3aR/C5aR can regulate immune responses by translating information gathered by complement fluid phase sensors into cellular responses. C3aR/C5aR have been reported to activate the autophagy inhibitor mTOR in cells. While depletion of autophagy in recipient DCs aggravates GVHD severity, and enhancing autophagy represents as a promising approach for treating immunological diseases, it is not known how escalation of autophagy regulates DC-functionality in GVHD pathogenesis. In the current study, we found that deficiency of C3aR/C5aR signaling significantly upregulated mTOR-independent autophagy in DCs (Figure 1B), which is associated with increased apoptosis, and reduced activation and antigen presenting capacity of C3aR/C5aR-/-DCs (Figure 1, A-C).
To evaluate the role of C3aR/C5aR in modulating DC function in GVHD pathogenesis, we compared the severity of GVHD in WT versus C3aR/C5aR-/- recipients using murine models of allo-HCT. C3aR/C5aR-/- recipients developed less severe GVHD than their WT counterparts, reflected by a significantly higher rate of survival and lower clinical scores (Figure 1D). Donor T-cell survival and Th1-differentiation were significantly decreased, whereas Treg generation was increased in C3aR/C5aR-/- recipients. Glycolytic activity of donor T cells was significantly abrogated in the absence of recipient C3aR/C5ar (Figure 1E). Donor T-cells expressed lower levels of chemokine receptors, CXCR3 and CCR6, which were likely contributed to the decreased migration of the effector T cells into GVHD target organs of C3aR/C5aR-/- recipients. Using chimeric recipients in which either the hematopoietic or non-hematopoietic compartment is deficient for C3aR/C5aR, we found that C3aR/C5aR in the recipient hematopoietic cells was primarily responsible for donor T-cell response and pathogenicity in GVHD. Transfusion of C3aR/C5aR-/- DCs along with allo-HCT mitigated GVHD severity, indicating that C3aR/5aR signaling in DCs plays a predominant role in the pathogenesis of GVHD. Elevated autophagy was required for the suppressive effect of C3aR/C5aR-/- DCs on GVHD as autophagy inhibitor chloroquine (CQ) reverted the GVHD-suppressive effect of C3aR/C5aR-/-DCs (Figure 1F). For translational purpose, we evaluated the therapeutic potential of a combination of C3aR and C5aR antagonists, and found that pharmacological blockade of C3aR/C5aR could effectively reduce GVHD severity while sparing GVL effect through persevering CD8 cytolytic activity.
The current study reveals for the first time that escalation of autophagy in DCs is an effective therapeutic approach for the control of GVHD, and establishes and interaction between complement and autophagy in allo-HCT. We also provide a novel strategy of enhancing autophagy as a DC-based vaccination strategy for the control of GVHD after transplantation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal